The Australasian Pelvic Floor Procedure Registry (APFPR) offers an important clinician-led opportunity for surgeons and healthcare operators to contribute to the safety and quality of pelvic floor device surgery in Australia. The data collected will ultimately create a powerful dataset. Once the dataset is mature, the benefits include: the ability to track patients, complications and devices inserted; provision of regular, timely reports regarding pelvic device procedure activity and outcomes to inform clinical practice; and opportunity to compare outcomes of pelvic device procedure with similar international datasets.
FREQUENTLY ASKED QUESTIONS (FAQS)
APFPR has been designed to minimise your participation overheads and time spent on data entry and once the data set has matured it will deliver valuable tools that your practice can use.
The APFPR does not yet integrate with any electronic health record (EHR) systems but it is a user-friendly, web-based data entry tool designed to reduce the burden of data entry.
You can view a summary of the data items we collect here.
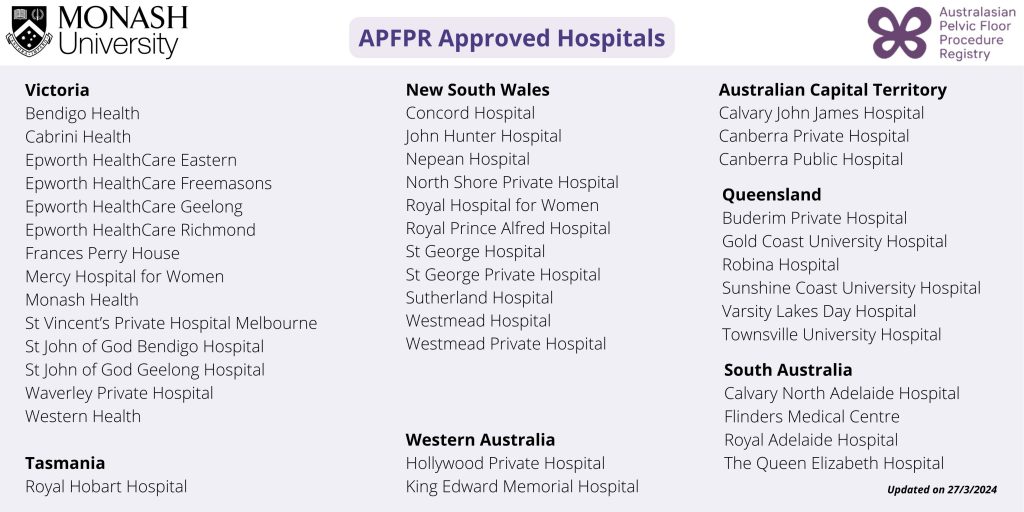
If you wish to view which hospitals are already approved to contribute data, please click here.
Surgeons can access information directly from the database about their own patients (identified), including basic reports.
Surgeon-level reports that the APFPR will securely provide to each participating surgeon will be a report of aggregate outcomes of their patients, but data in this format will not identify patients.
Participating sites will receive reports of their own aggregate data but no identifiable surgeon or patient data.
As the dataset matures these reports will be risk-adjusted and benchmarked so that surgeons will be able to monitor patient care and evaluate patient outcomes.
The APFPR captures data for patients undergoing stress urinary incontinence (SUI) procedures involving an implant, as well as native tissue procedures. We also capture data for patients undergoing Pelvic Organ Prolapse procedures.
To view a list of included procedures, please click here.
Please contact the APFPR Project Team to determine what pelvic floor procedures are being accepted.
Once this is determined, hospital ethics and governance approval is required prior to including a patient on the registry.
When this is obtained, patients eligible for participation can be approached, provided with a patient flyer and registered on the database.
At the time of surgery and post-operative visits, information about their clinical history, pelvic floor status, operation and complications can be provided either directly into the registry database, or via paper collection forms.
In the future the APFPR will work with electronic record providers to integrate APFPR data items into electronic medical record systems.
Access to data collected by the registry is strictly controlled by the protocol, data access policy, HREC approval and is overseen by the APFPR Steering Committee.
Protocols are in place to ensure that the highest levels of privacy and safety are maintained.
Surgeons can access information directly from the database about their own patients (identified), including basic reports.
Participating sites will receive reports of their own aggregate data but no identifiable surgeon or patient data.
Patients can apply to the Registry Coordinator to access their own data if required and researchers can apply to the Steering Committee to access data from the database.
However, all requests must be approved by the APFPR and be compliant with the APFPR Data Access and Publication Policy.
The APFPR plays an important part in future health outcomes of Australians because it aims to record information that will ultimately improve quality of care and increase safety for patients undergoing procedures that involve medical devices such as pelvic surgical mesh. From January 2024, the APFPR is also capturing outcomes for the following SUI native tissue procedures:
- Autologous Fascial sling
- Burch Colposuspension
The surgical and patient reported outcomes of mesh procedures, including initial procedures and those requiring revision or mesh removal, are used to provide safety and quality of care reports to hospitals, surgeons, the TGA (Therapeutic Goods Administration) and government.
As more data is collected more information can be analysed to not only inform surgical best practice, but to inform patients who are considering pelvic surgery with mesh to better understand the benefits and potential risks of these procedures.
The APFPR also produces public annual (aggregate data) reports and patient-facing infographics about registry data.
A quick summary of how the process works for clinicians can be found here.
A snapshot of the data items captured can be found here.
Monash University has established and managed clinical registries for more than 20 years.
Monash currently operates nearly 40 state and national registries.
Monash University is accredited at the highest levels of data security and governance systems for sensitive data.
The APFPR complies with State, Territory and Commonwealth privacy laws, with your data permanently stored on infrastructure located in Australia.
Data stored on this infrastructure is backed up daily and accessed by trained APFPR personnel only.
All traffic between the data collector’s personal device, the web server(s), the database server(s), and the file server(s) is encrypted.
External access to information from the APFPR must adhere to strict protocols and procedures in line with the APFPR data access policy.
Registry data will be made available to external researchers (upon Steering Committee and ethics approval if applicable) but only after all identifiable patient and surgeon information has been removed.
Additionally, the APFPR Steering Committee will review all publications prior to submission.
Registry data can be accessed for research purposes in line with the conditions set out in the APFPR’s Data Access and Publication Policy. A Data Access Request form needs to be completed and sent to the APFPR Coordinating team. The request will be tabled for review by the APPFR Steering Committee. If approved, data will be de-identified at the patient, surgeon and hospital level.
Any enquiries regarding data access should be directed to the APFPR Project Team by calling 1800 571 093 (toll free) or emailing apfpr@monash.edu.
Please contact the APFPR Project Team by calling 1800 571 093 (toll free) or emailing apfpr@monash.edu.